





Preview
基本信息产品详情证书
基本信息
| 型号 | MY-01 |
| 认证 | API:应用程序编程接口 CE:欧洲统一认证 FDA:美国食品药品监督管理局 GMP:良好生产规范 ISO:国际标准化组织 UL:保险商试验室(安全检测机构) |
| 条件 | 新 |
| 定制的 | 定制的 |
| 检测范围 | 激素、代谢物、核酸、蛋白质、小分子 |
| 材料兼容性 | 胶囊、晶体、颗粒、中草药、液体、粉末、药片 |
| 可移植性 | 基台 |
| 电源 | 电池驱动 |
| 灵敏度 | 高度敏感 |
| 技术 | 红外光谱法 |
| 用户界面 | 软件接口 |
| 电压 | 220V至380V |
| 功能 | 检查 |
| 维度 | 定制的 |
| 用法 | 药品检验 |
| 材料 | 304/316不锈钢 |
| 运输包 | 木制包装 |
| 规格 | 定制的 |
| 商标 | 玛丽亚 |
| 起源 | 中国 |
| 生产能力 | 年产量五十万件 |
| 包装尺寸 | 400.00cm乘400.00cm乘400.00cm |
| 包装总重量 | 一百万千克 |
| 包装尺寸 | 400.00cm乘400.00cm乘400.00cm |
| 包装总重量 | 一百万千克 |
产品详情
Marya China supplier pharmaceutical inspection device isolator system for sterilization
Description:
Sterile Isolator
As a decontamination equipment separating operators and machines and complying with the requirements of Class A environment, the sterile isolator is mainly used for the protection of sterile testing, sterile product dispensing and other key process. It can reduce the background or environment requirements, improve the reliability of inspection results, meet the requirements of China GMP and Pharmacopoeia, EU GMP/FDA and cGMP/USP-NF, and can carry out "continuous" or "batch" operation.
Description:
Sterile Isolator
As a decontamination equipment separating operators and machines and complying with the requirements of Class A environment, the sterile isolator is mainly used for the protection of sterile testing, sterile product dispensing and other key process. It can reduce the background or environment requirements, improve the reliability of inspection results, meet the requirements of China GMP and Pharmacopoeia, EU GMP/FDA and cGMP/USP-NF, and can carry out "continuous" or "batch" operation.
Preview

Preview

Preview
Characteristic and Performance:
1. New VHPS technology:control the concentration and saturation of H2O2; good sterilization reproducibility; shorten the sterilization time of the chamber;2. Modular design:including transfer chamber, operation chamber, bacteria collector and other functional modules and configurations; easy to install and transport;3. Intelligent control system:Siemens PLC+IPC control system; intelligent alarms of pressure, H2O2 solution concentration, temperature and humidity, air speed and other monitoring parameters; the system has multi-level access control; comply with FDA 21 CFR part 11 requirements;4. CDCV service:sterilization cycle development, validation studies and services;5. Wireless glove leakage detector:integrated or wireless glove leakage detector is optional;6. Energy saving and consumption reduction:optimized design of airflow handling system; energy consumption of eight-hour continuous operation reduces by 20%;7. Real-time monitoring of door status: it can monitor the door opening online and continuously monitor the airtight seal of the door;8. High-quality manufacturing materials: the inner chamber is made of 316L or PTFE, and the chamber polishing grade is 0.4μm~0.6μm;9. Decomposition filter:the air exhaust module can be equipped with a decomposition filter can effectively shorten the degradation time after H2O2 sterilization, and effectively reduce the concentration level of residues;10. Simple maintenance: the maintenance surface is on the front, and the space required for the laboratory is not big.
Characteristic and Performance:1. New VHPS technology:control the concentration and saturation of H2O2; good sterilization reproducibility; shorten the sterilization time of the chamber;2. Modular design:including transfer chamber, operation chamber, bacteria collector and other functional modules and configurations; easy to install and transport;3. Intelligent control system:Siemens PLC+IPC control system; intelligent alarms of pressure, H2O2 solution concentration, temperature and humidity, air speed and other monitoring parameters; the system has multi-level access control; comply with FDA 21 CFR part 11 requirements;4. CDCV service:sterilization cycle development, validation studies and services;5. Wireless glove leakage detector:integrated or wireless glove leakage detector is optional;6. Energy saving and consumption reduction:optimized design of airflow handling system; energy consumption of eight-hour continuous operation reduces by 20%;7. Real-time monitoring of door status: it can monitor the door opening online and continuously monitor the airtight seal of the door;8. High-quality manufacturing materials: the inner chamber is made of 316L or PTFE, and the chamber polishing grade is 0.4μm~0.6μm;9. Decomposition filter:the air exhaust module can be equipped with a decomposition filter can effectively shorten the degradation time after H2O2 sterilization, and effectively reduce the concentration level of residues;10. Simple maintenance: the maintenance surface is on the front, and the space required for the laboratory is not big.
1. New VHPS technology:control the concentration and saturation of H2O2; good sterilization reproducibility; shorten the sterilization time of the chamber;2. Modular design:including transfer chamber, operation chamber, bacteria collector and other functional modules and configurations; easy to install and transport;3. Intelligent control system:Siemens PLC+IPC control system; intelligent alarms of pressure, H2O2 solution concentration, temperature and humidity, air speed and other monitoring parameters; the system has multi-level access control; comply with FDA 21 CFR part 11 requirements;4. CDCV service:sterilization cycle development, validation studies and services;5. Wireless glove leakage detector:integrated or wireless glove leakage detector is optional;6. Energy saving and consumption reduction:optimized design of airflow handling system; energy consumption of eight-hour continuous operation reduces by 20%;7. Real-time monitoring of door status: it can monitor the door opening online and continuously monitor the airtight seal of the door;8. High-quality manufacturing materials: the inner chamber is made of 316L or PTFE, and the chamber polishing grade is 0.4μm~0.6μm;9. Decomposition filter:the air exhaust module can be equipped with a decomposition filter can effectively shorten the degradation time after H2O2 sterilization, and effectively reduce the concentration level of residues;10. Simple maintenance: the maintenance surface is on the front, and the space required for the laboratory is not big.
Characteristic and Performance:1. New VHPS technology:control the concentration and saturation of H2O2; good sterilization reproducibility; shorten the sterilization time of the chamber;2. Modular design:including transfer chamber, operation chamber, bacteria collector and other functional modules and configurations; easy to install and transport;3. Intelligent control system:Siemens PLC+IPC control system; intelligent alarms of pressure, H2O2 solution concentration, temperature and humidity, air speed and other monitoring parameters; the system has multi-level access control; comply with FDA 21 CFR part 11 requirements;4. CDCV service:sterilization cycle development, validation studies and services;5. Wireless glove leakage detector:integrated or wireless glove leakage detector is optional;6. Energy saving and consumption reduction:optimized design of airflow handling system; energy consumption of eight-hour continuous operation reduces by 20%;7. Real-time monitoring of door status: it can monitor the door opening online and continuously monitor the airtight seal of the door;8. High-quality manufacturing materials: the inner chamber is made of 316L or PTFE, and the chamber polishing grade is 0.4μm~0.6μm;9. Decomposition filter:the air exhaust module can be equipped with a decomposition filter can effectively shorten the degradation time after H2O2 sterilization, and effectively reduce the concentration level of residues;10. Simple maintenance: the maintenance surface is on the front, and the space required for the laboratory is not big.
Preview

Preview

Preview

Preview
Technical Data
MOC
316 stainless steel wire drawing board
Sealing
The leakage rate of the cabin is less than 5% within 10min under the pressure of 500Pa
Wind speed
0.35~0.65m/s, adjustable wind speed, keep 20~60Pa during aseptic operation, maintain the pressure under static conditions to a set value of not less than 10Pa
Automatic removal efficiency
<1PPM, 10~20min
Cleanliness
Degree
Class A
Power source
AC220V±22V, 50Hz±1Hz
Size
Standard inner compartment 1800*710*850Standard outer dimensions: 1900*800*2200, can be customized according to requirements
Verification content
Sterilization efficiency: biological indicator, chemical test
MOC
316 stainless steel wire drawing board
Sealing
The leakage rate of the cabin is less than 5% within 10min under the pressure of 500Pa
Wind speed
0.35~0.65m/s, adjustable wind speed, keep 20~60Pa during aseptic operation, maintain the pressure under static conditions to a set value of not less than 10Pa
Automatic removal efficiency
<1PPM, 10~20min
Cleanliness
Degree
Class A
Power source
AC220V±22V, 50Hz±1Hz
Size
Standard inner compartment 1800*710*850Standard outer dimensions: 1900*800*2200, can be customized according to requirements
Verification content
Sterilization efficiency: biological indicator, chemical test
Preview

Preview

Preview

Preview
About Us
Preview
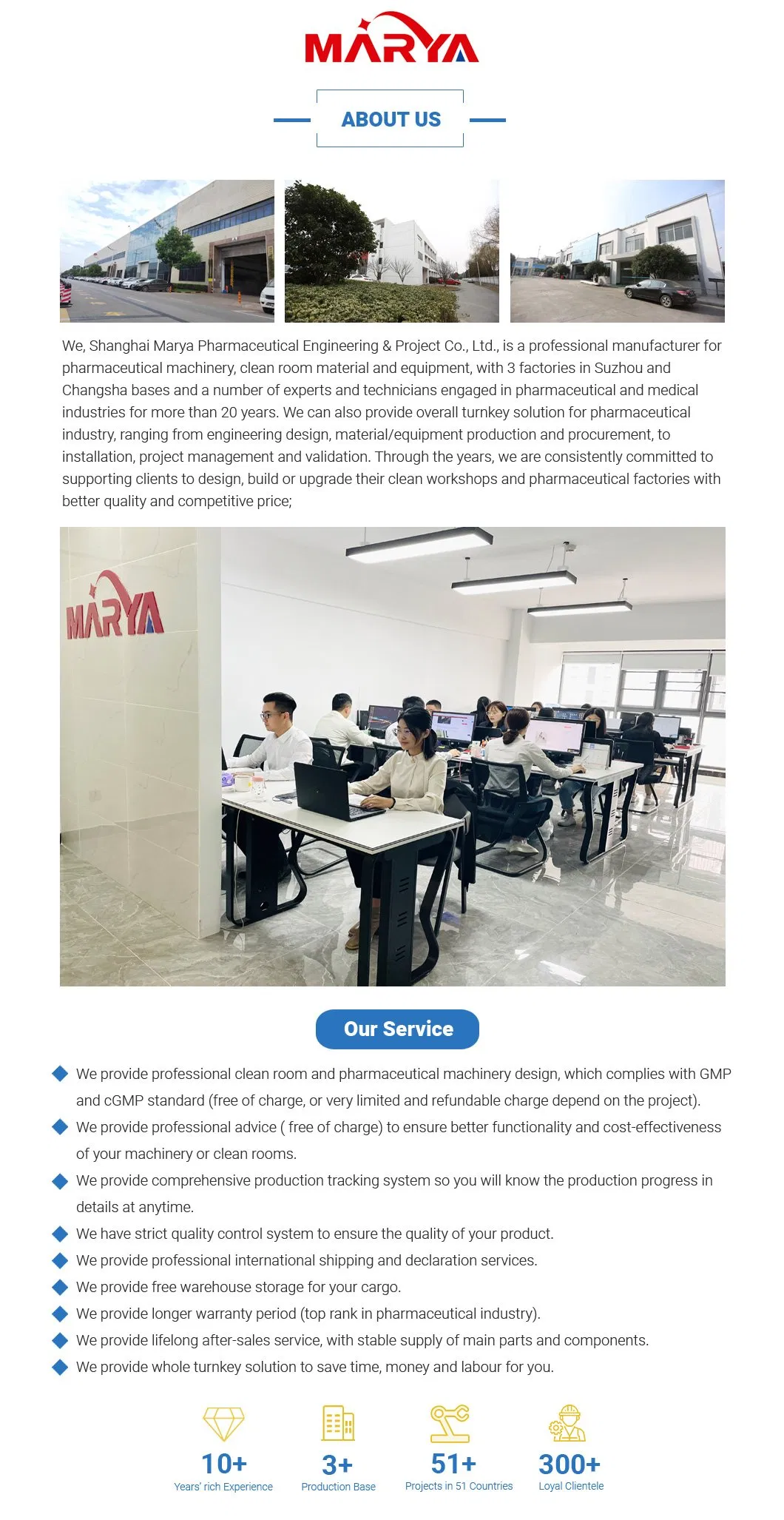
Preview
Workshop
Preview

Preview
Certificate
Preview

Preview
Project case
Preview

Preview
Our Customer
Preview

Preview
Exhibition & Our Team
Preview

Preview

Preview
Packaging & Shipping
Preview

Preview
证书
标题:CE认证洁净室和HVAC系统

Preview


 上海
上海  已认证
已认证


