




Preview
基本信息产品详情证书
基本信息
| 型号编号 | LFP-400 |
| 阴极材料 | LFP |
| 类型 | 电解质 |
| 交通包 | 包 |
| 规格 | LFP |
| 商标 | stonepack |
| 起源 | 中国 |
| 商品编码 | 8507600090 |
| 生产能力 | 每年二十万吨 |
产品详情
Product Description
Preview

Preview
Preview
Safety, Reliable
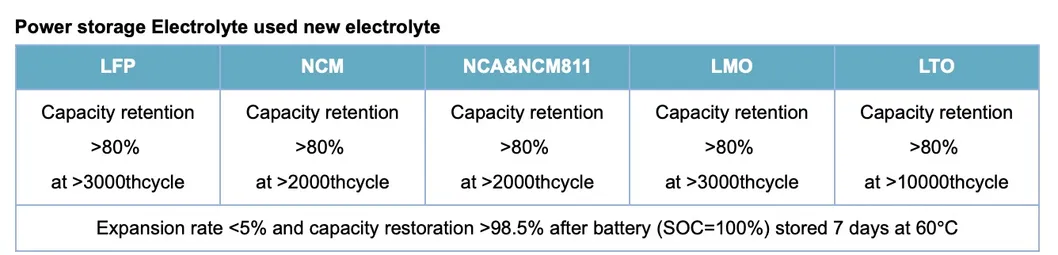
Pure electric vehicles (EV) and energy storage power station (ES) is the future trend based on environmental protection and sustainable development. Electrolyte can meet the requidiments for Ithium-lon battery, such as long life, high security, etc
Lithium metal batteries:
Basic Principles of Lithium Batteries
Basic Principles of Lithium Batteries
Lithium metal batteries generally use manganese dioxide as the positive electrode material, metal lithium or its alloy metal as the negative electrode material, and use non aqueous electrolyte solutions.Discharge reaction: Li+MnO2=LiMnO2
Lithium ion battery:
Lithium ion batteries generally use lithium alloy metal oxides as the positive electrode material, graphite as the negative electrode material, and non aqueous electrolytes.The reaction that occurs on the charging positive electrode is
LiCoO2=Li (1-x) CoO2+xLi++xe - (electronic)
The reaction that occurs on the charging negative electrode is
6C+xLi++xe- = LixC6
Total reaction of rechargeable battery: LiCoO2+6C=Li (1-x) CoO2+LixC6
Features:
1.High capacity
2.Rate performance
3.Long cycle life
Pure electric vehicles (EV) and energy storage power station (ES) is the future trend based on environmental protection and sustainable development. Electrolyte can meet the requidiments for Ithium-lon battery, such as long life, high security, etc
Lithium metal batteries:
Basic Principles of Lithium Batteries
Basic Principles of Lithium Batteries
Lithium metal batteries generally use manganese dioxide as the positive electrode material, metal lithium or its alloy metal as the negative electrode material, and use non aqueous electrolyte solutions.Discharge reaction: Li+MnO2=LiMnO2
Lithium ion battery:
Lithium ion batteries generally use lithium alloy metal oxides as the positive electrode material, graphite as the negative electrode material, and non aqueous electrolytes.The reaction that occurs on the charging positive electrode is
LiCoO2=Li (1-x) CoO2+xLi++xe - (electronic)
The reaction that occurs on the charging negative electrode is
6C+xLi++xe- = LixC6
Total reaction of rechargeable battery: LiCoO2+6C=Li (1-x) CoO2+LixC6
Features:
1.High capacity
2.Rate performance
3.Long cycle life
Preview

Preview
Preview
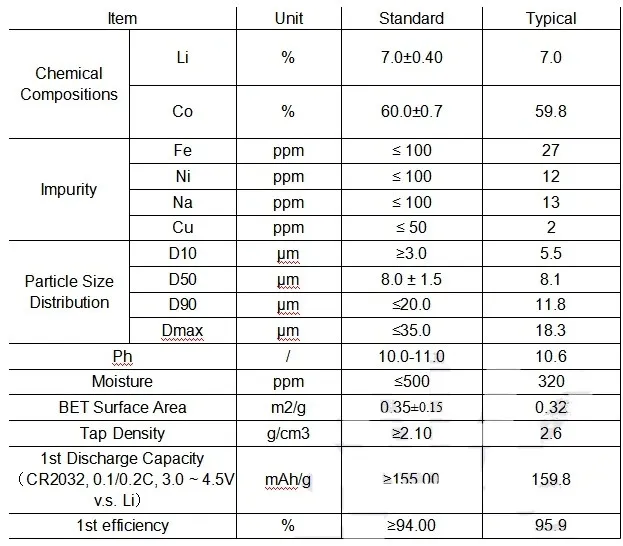
1.Full set of lithium battery materials,including :
LiMn2O4,LTO,LiNiMnCoO2(NMC),LiCoO2,Graphite(MCMB)and other cathode&anode battery materials;Aluminum foil,copper foils,battery separator,etc.
2.Full set of lithium battery equipments,for example:
mixing machine --coating machine--oven--rolling machine--welding machine--slitting / cutiing machine --winding machine--sealed machine,etc.
3.Full set of lithium battery technology.
we can design the laboratory and production line,according to customer's request.
LiMn2O4,LTO,LiNiMnCoO2(NMC),LiCoO2,Graphite(MCMB)and other cathode&anode battery materials;Aluminum foil,copper foils,battery separator,etc.
2.Full set of lithium battery equipments,for example:
mixing machine --coating machine--oven--rolling machine--welding machine--slitting / cutiing machine --winding machine--sealed machine,etc.
3.Full set of lithium battery technology.
we can design the laboratory and production line,according to customer's request.
Preview
证书
标题:供应商评估报告

Preview


 上海
上海  已认证
已认证

